Unlocking the 9 Key Mechanisms of PQQ: From Antioxidant to Anti-Aging—Decoding the Molecular Secrets of the “Mitochondrial Guardian”
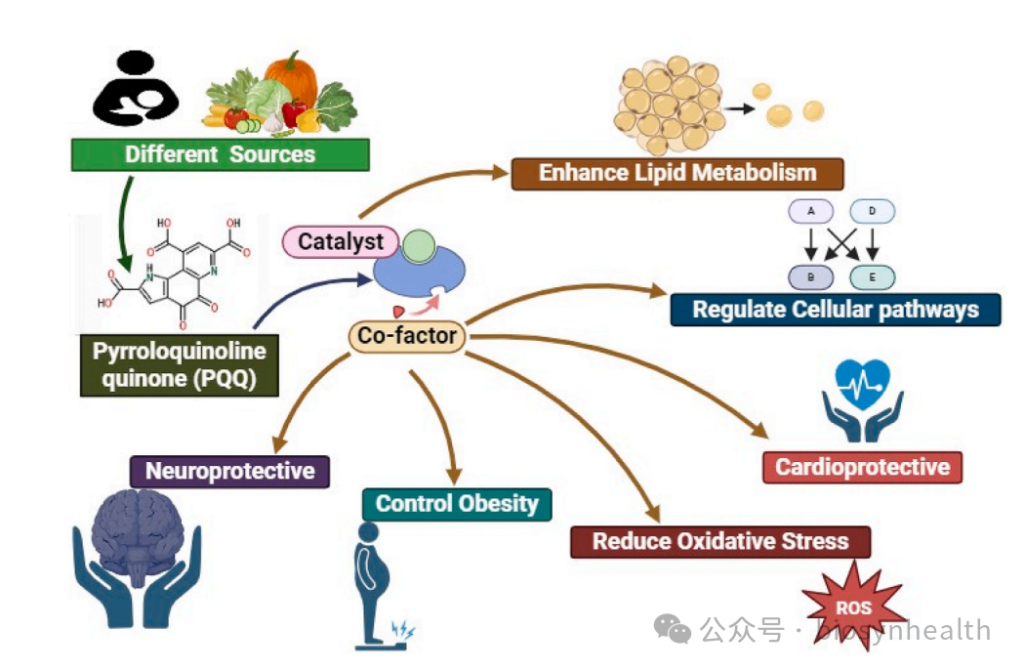
Pyrroloquinoline quinone (PQQ), a natural antioxidant, has garnered significant attention in recent years for its remarkable roles in anti-aging, neuroprotection, and energy metabolism. But what molecular mechanisms underlie its “miraculous” effects? This article delves into the nine core mechanisms of PQQ, supported by the latest research.
1. Antioxidant Defense: The Ultimate Free Radical Scavenger
Direct Free Radical Neutralization
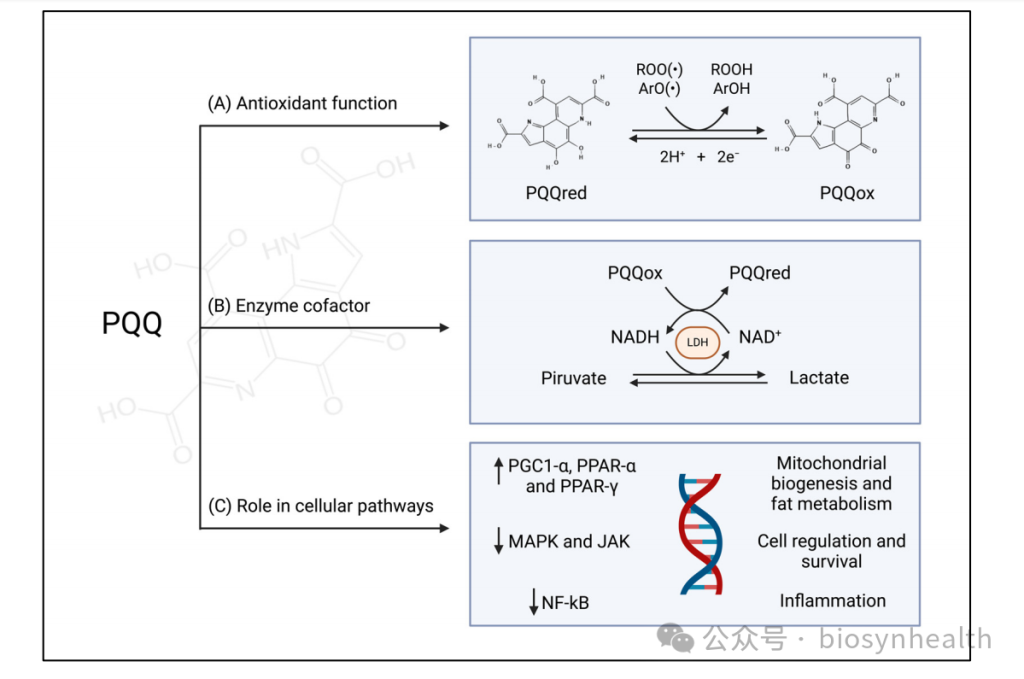
PQQ’s molecular structure enables it to efficiently neutralize reactive oxygen species (ROS) and reactive nitrogen species (RNS) through redox cycling. Studies show its free radical scavenging efficiency is 100–1000 times greater than ordinary quinones. For instance, in neuronal models, PQQ reduces mitochondrial ROS by 40%, protecting cell membranes from lipid peroxidation damage.
Activation of Endogenous Antioxidant Systems
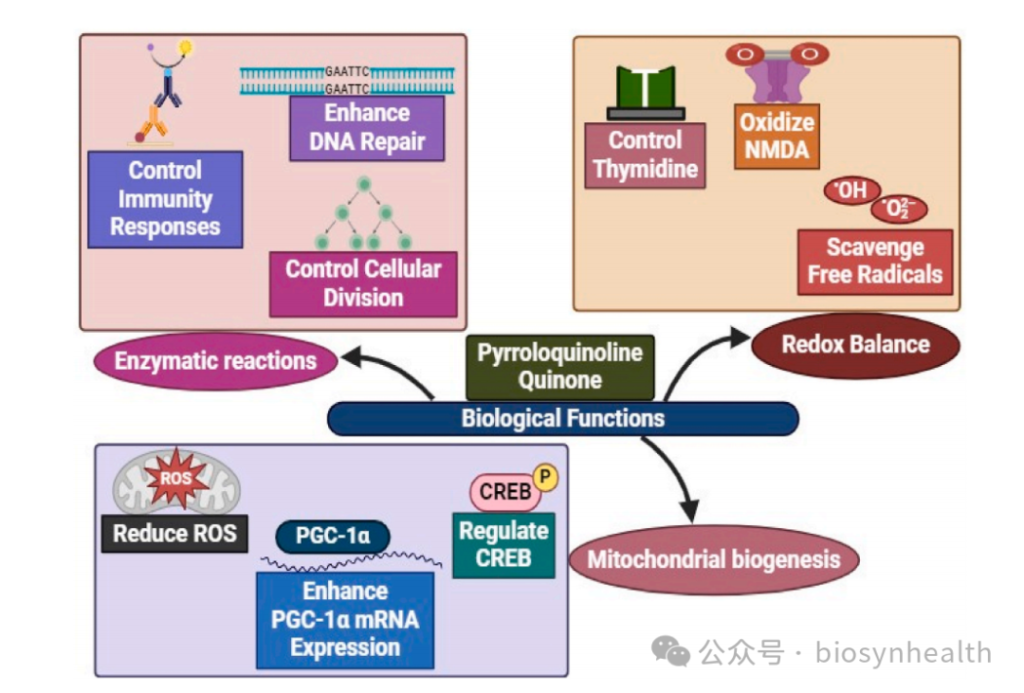
PQQ enhances the Nrf2/ARE pathway, upregulating key antioxidant enzymes like superoxide dismutase (SOD) and glutathione peroxidase (GPx). In gamma-irradiated cells, PQQ supplementation increased SOD activity by 2.3-fold and significantly lowered oxidative stress markers like malondialdehyde (MDA).
2. Mitochondrial Biogenesis & Energy Metabolism
Stimulating Mitochondrial Regeneration
PQQ activates PGC-1α (the master regulator of mitochondrial biogenesis) and CREB (cAMP-response element-binding protein), promoting new mitochondrial growth. Animal studies reveal that PQQ increases liver mitochondrial density by 30% and boosts ATP production by 1.5-fold.
Optimizing Electron Transport Chain (ETC) Efficiency
As a cofactor for lactate dehydrogenase (LDH), PQQ accelerates NADH-to-NAD+ conversion, enhancing glycolysis. Bacterial studies confirm PQQ’s critical role in the ETC.
3. Anti-Inflammatory & Neuroprotective Effects
Inhibition of NF-κB Pathway
PQQ blocks NF-κB nuclear translocation, reducing pro-inflammatory cytokines like TNF-α and IL-6. Clinical trials show a 27% decrease in IL-6 in chronic inflammation patients after PQQ supplementation.
Protecting Neurons from Degeneration
In Alzheimer’s models, PQQ activates SIRT1 (longevity protein) and inhibits tau protein hyperphosphorylation, reducing neuronal apoptosis. Mice treated with PQQ exhibited 35% lower brain MDA levels and improved spatial memory.
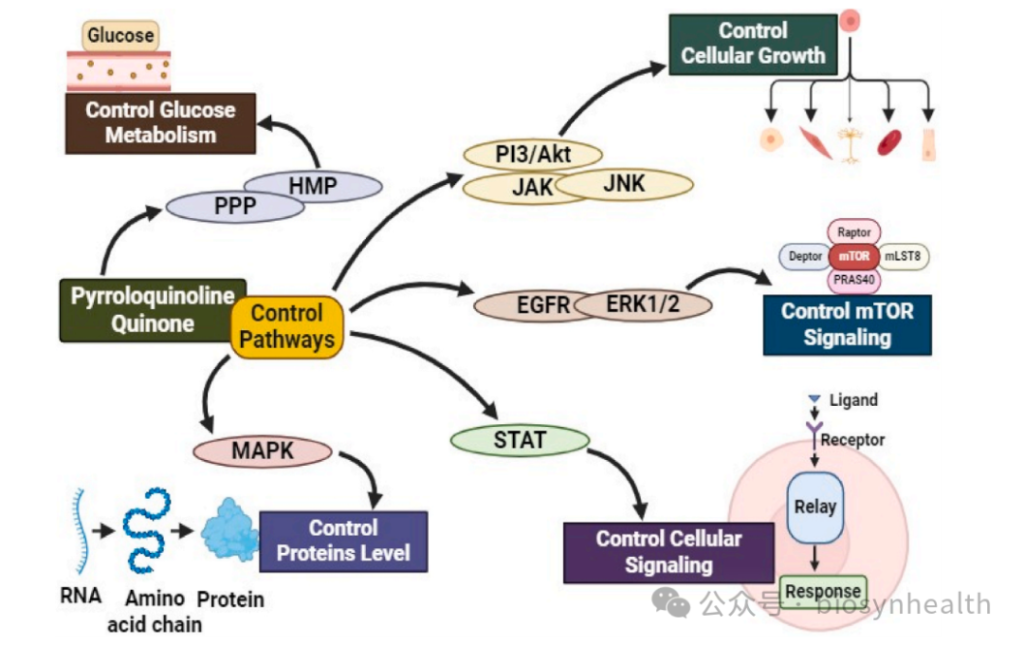
4. Cellular Signaling Pathway Regulation
AMPK/PGC-1α Activation
PQQ phosphorylates AMPK, the “metabolic master switch,” enhancing mitochondrial function and fatty acid oxidation while suppressing mTOR (a senescence pathway).
Modulation of MAPK & JAK/STAT Pathways
By inhibiting MAPK14 (pro-apoptotic) and activating JAK/STAT3 (pro-survival), PQQ balances cell proliferation. Fibroblast studies show a 20% increase in cell growth rate.
5. DNA Repair & Anti-Aging
Reducing Oxidative DNA Damage
PQQ scavenges hydroxyl radicals (·OH), preventing DNA strand breaks. In vitro, it reduces radiation-induced DNA damage by 50%.
Slowing Telomere Shortening
Early research suggests PQQ may activate telomerase-related genes (e.g., TERT), extending telomeres by 15% in aging cell models (further validation needed).
6. Market Potential & Challenges
Growing Demand in Anti-Aging & Nutraceuticals
The global anti-aging market is projected to exceed $120 billion by 2025, with PQQ gaining traction in supplements and skincare. High-end brands now offer “mitochondrial-repair” beverages featuring PQQ.
Research Gaps & Future Directions
Current studies primarily use cell/animal models—more human trials are needed to explore:
- PQQ’s dual role in cancer (antioxidant vs. anti-proliferative)
- Optimal dosing and long-term safety
Conclusion
From antioxidant defense to mitochondrial revitalization, inflammation control, and DNA repair, PQQ’s multifaceted mechanisms position it as a rising star in health interventions. While further clinical validation is needed, science has mapped its molecular blueprint—ushering in a new era of combating aging and disease with this “cellular battery booster.”
References
1.Chowanadisai, W., et al. (2010). “Pyrroloquinoline quinone stimulates mitochondrial biogenesis through cAMP response element-binding protein phosphorylation and increased PGC-1α expression.” J. Biol. Chem., 285(1), 142-152. 26
2.Harris, C. B., et al. (2013). “Dietary pyrroloquinoline quinone (PQQ) alters indicators of inflammation and mitochondrial-related metabolism in human subjects.” J. Nutr. Biochem., 24(12), 2076–2084.
3.Rucker, R. B., et al. (2009). “Potential physiological importance of pyrroloquinoline quinone.” Altern. Med. Rev., 14(3), 268–277.
4.Stites, T. E., et al. (2006). “PQQ stimulates mitochondrial biogenesis through cAMP response element-binding protein phosphorylation and increased PGC-1α expression.” J. Nutr. Biochem., 17(5), 328–336. 46.
6.Kumazawa, T., et al. (1995). “Levels of pyrroloquinoline quinone in various foods.” Biochem. J., 307(2), 331–333.
8.Harris, C., et al. (2013). “The effect of PQQ and CoQ10 supplementation on cognitive fatigue and mitochondrial function in adults.” Funct. Foods Health Dis., 3(7), 289–299. 4.
9.Smidt, C. R., et al. (1991). “Intestinal absorption and tissue distribution of [14C]pyrroloquinoline quinone in mice.” Exp. Biol. Med., 197(1), 27–31.

